Automated pharma compliance platform

AI-pharma assistance
AI-powered regulatory
management system
RoboReg simplifies drug registration and regulatory submissions in Canada using AI and RPA. It auto-fills eCTD documents, integrates via API, and supports team collaboration—reducing errors, improving compliance, and speeding up approvals.
RoboReg is operating as an AI-powered Regulatory. Information Management System for streamlining and accelerating registration and submission of new drugs by pharmaceutical and medical device companies to regulatory authorities Canada
Roboreg’s vision is to help Pharmaceutical and Medical. Device Manufacturers to launch new products within shortest possible time to save time, cost & lives.
Why regulatory submissions are broken
Regulatory teams rely on spreadsheets, emails, and manual processes that slow down submissions.
Human error in formatting, version control, or data entry can result in costly delays or rejections.
Regulatory, clinical, and quality teams work separately, causing miscommunication, duplicated efforts, and errors.
Keeping up with evolving global guidelines like eCTD requires time, expertise, and constant updates.

The smarter way to manage regulatory submissions
RoboReg uses AI and automation to simplify regulatory submissions, reduce errors, and speed up drug approvals.

Automatically insights from regulatory documents with NLP—no more manual scanning or tagging.
RoboReg aligns your documents with Health Canada and FDA standards, including eCTD formatting.
A single cloud-based platform where regulatory, legal, and compliance teams can work in sync.
Stay up-to-date with the latest guidelines and leverage data from past successful submissions.
What you gain with RoboReg
RoboReg helps pharma teams work faster, cut errors, and stay compliant—so you can focus on bringing products to market.
Automate critical submission steps to
speed up the approval process. Get your products to market faster and stay ahead
of the competition.
AI and robotic process automation minimize manual mistakes in document preparation and formatting. This reduces costly rejections and delays.
Streamline workflows to cut labor expenses and reduce costly delays that can cost millions. Save valuable resources while improving overall efficiency.
Automatically update your submissions
to stay aligned with evolving regulatory requirements. Be audit-ready at all times with less effort.
Simplify workflows to reduce time spent
on approvals and follow-ups. Empower
your team to focus on innovation instead
of paperwork.
Unify regulatory, clinical, and quality teams seamlessly on a single platform. Improve communication and reduce duplication of work across departments.
How RoboReg automates your regulatory workflow
RoboReg simplifies the regulatory submission process with automation, AI, and smart collaboration.
Start by launching a new regulatory submission from your dashboard.

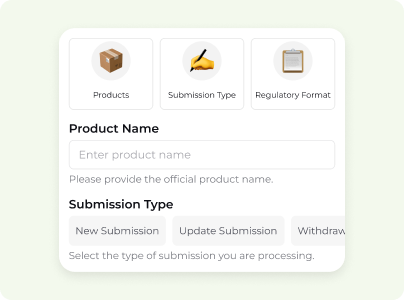
Fill in structured forms with product info, submission type, and regulatory format.


Easily import regulatory files or connect via API. RoboReg supports a wide range of formats and data sources.
What makes RoboReg a game-changer
RoboReg uses AI and automation to simplify regulatory submissions, reduce errors, and speed up drug approvals.
Automatically extract, organize, and analyze regulatory data using natural language processing—no manual review.
Eliminate repetitive tasks like form filling and formatting. Let bots handle submissions while you focus on strategy.
Keeping up with evolving global guidelines like eCTD requires time, expertise, and constant updates.
What our clients say
Trusted by pharma leaders in Canada.

Emily Chen
Director of Lupin Pharm, Canada

“ROBOREG has completely transformed how we manage regulatory submissions. What used to take weeks of manual work—organizing documents, formatting to eCTD, and chasing down team inputs—can now be done in a fraction of the time. The AI and automation features are not only smart but incredibly accurate.”

Emily Chen
Director of Lupin Pharm, Canada

“ROBOREG has completely transformed how we manage regulatory submissions. What used to take weeks of manual work—organizing documents, formatting to eCTD, and chasing down team inputs—can now be done in a fraction of the time. The AI and automation features are not only smart but incredibly accurate.”

Emily Chen
Director of Lupin Pharm, Canada

“ROBOREG has completely transformed how we manage regulatory submissions. What used to take weeks of manual work—organizing documents, formatting to eCTD, and chasing down team inputs—can now be done in a fraction of the time. The AI and automation features are not only smart but incredibly accurate.”

Emily Chen
Director of Lupin Pharm, Canada

“ROBOREG has completely transformed how we manage regulatory submissions. What used to take weeks of manual work—organizing documents, formatting to eCTD, and chasing down team inputs—can now be done in a fraction of the time. The AI and automation features are not only smart but incredibly accurate.”
Join our research initiative
Help us understand your needs better by participating in our comprehensive survey.

Quick and easy
Our survey is short and simple, taking only 5–10 minutes to complete.

Make an impact
Your feedback helps us improve ROBOREG to better meet your needs.

Exclusive access
Participants may receive early access to new features and improvements.
The RoboReg team
The RoboReg team is a group of experts in pharma, AI, and compliance, dedicated to transforming regulatory workflows.

Sudhir Chandra Biswas
Chief Executive Officer

Md Mahbub Aleem
Chief Operating Officer

Mohammad Mahmudul
Technical Advisor

Muhammad Kader
Overseas Advisor
Read our articles
Explore expert insights and industry updates in our latest articles.
Health Canada regulatory submissions are often described as complex, time-consuming, and risky and for good
In the rapidly evolving landscape of pharmaceutical and medical device development, maintaining
Frequently asked questions
1. What is Roboreg?
Roboreg is an AI-powered platform that simplifies regulatory compliance for businesses. Instead of spending hours navigating complex rules, you can automate key processes and stay compliant with confidence.
2. Who can benefit from Roboreg?
Roboreg is designed for startups, SMEs, and enterprises that operate in regulated industries in Canada. Whether you’re in finance, healthcare, tech, or legal services, Roboreg helps you reduce risk and save time.
3. How does Roboreg ensure accuracy in compliance?
Our system continuously monitors regulatory updates and applies AI-driven checks. This means you get real-time insights and alerts whenever rules change, so your business always stays ahead.
4. Is Roboreg difficult to use?
Not at all. Roboreg is built with a simple, intuitive dashboard. Even if you don’t have a legal or compliance background, you can easily navigate and manage your requirements in just a few clicks.
5. How is Roboreg different from traditional compliance services?
Unlike costly manual consultants, Roboreg automates compliance at scale. You get instant updates, AI-driven reports, and ongoing monitoring, all at a fraction of the cost.
6. Is my data secure with Roboreg?
Yes. Data security is our top priority. Roboreg uses industry-standard encryption and follows strict Canadian data protection regulations to keep your information safe.


